Abstract
Background
Blinatumomab is a BiTE ® (bispecific T cell engager) molecule that engages patients' T cells to the CD19 antigen on lymphoid tumor cells. Blinatumomab administered as a 28-day continuous intravenous infusion (cIV) is approved in multiple regions for the treatment of R/R B-ALL in adults and children. Subcutaneous delivery may improve the convenience and satisfaction of patients with R/R B-ALL who are candidates for blinatumomab therapy. Here we report the results from the first cohort of adults with R/R B-ALL receiving SC blinatumomab.
Methods
In this ongoing multicenter, single arm, open-label, phase 1b dose-finding study (NCT04521231), patients received multiple cycles of SC blinatumomab. Each cycle included a treatment period and a treatment-free interval. In cohort 1, cycle 1, patients received a lower first dose of SC blinatumomab for several days followed by a higher dose multiple times weekly; in subsequent cycles, patients received the higher dose several times weekly during the treatment period. Bone marrow (BM) evaluation was performed on day 27 of each cycle.
Results
Six patients from cohort 1 were included in this June 22, 2021 data cutoff. Median age was 64 (range 38-83) years. The number of prior therapies ranged from 2-4. Two patients had disease refractory to primary therapy or salvage therapy, 2 patients relapsed after chemotherapy, and 2 patients relapsed after prior allogeneic hematopoietic stem cell transplant. Median BM blast count at study start was 85% (range 28%-95%). Only 1 patient had <50% BM blasts (BM blasts=28%). At enrollment, all patients had an ECOG score of 0-1. The median number of SC blinatumomab cycles initiated was 1 (range 1-3).
Preliminary pharmacokinetic results support the SC dosing intervals used in this study and potentially longer intervals. Exposures for SC doses were similar to the efficacious exposures of the approved cIV regimen: mean average concentrations at steady state of 215 and 853 pg/mL for the lower and higher SC dosing regimens of cohort 1, respectively, vs mean steady state concentrations of 228 and 616 pg/mL for 9 and 28 µg/day cIV dosing, respectively. The pharmacodynamic profile following SC blinatumomab of peripheral immune cell redistribution (circulating CD3+ and CD8+ CD69+ T cells), transient cytokine elevation (IL-6, IL-10, IFN-γ) and CD19+ B cell counts declining below the detection limit was consistent with the historical pharmacodynamic profile following cIV blinatumomab.
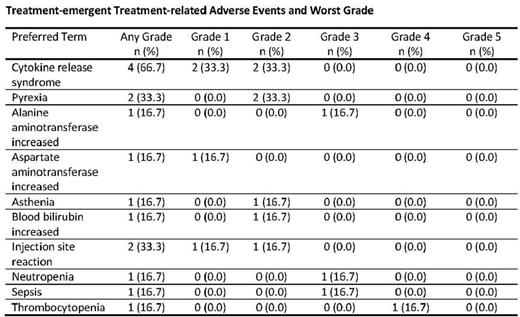
No grade ≥3 cytokine release syndrome events were reported (Table). One patient developed herpes encephalitis and experienced a grade 5 neurological event unrelated to blinatumomab; no other neurological events were reported. Two patients discontinued treatment because of adverse events (injection site reaction in patient with no response, hyperleukocytosis due to disease progression).
Three patients had complete hematological response (CR) with no measurable residual disease (MRD) (<10 -4) within 2 cycles and 1 patient had a morphological partial response (95% BM blasts at start of cycle 1 to 22% blasts on day 15). This patient discontinued on day 15 of cycle 1 after progression of extramedullary disease. At the time of the data cutoff, 2 patients remained on study. These patients had CR with no MRD.
Conclusions
In this phase 1b dose-finding study, SC blinatumomab has demonstrated encouraging anti-leukemia activity in heavily pretreated patients with R/R B-ALL. Pharmacokinetic and pharmacodynamic results support the use of SC dosing in this population. The safety profile was manageable and consistent with that reported for cIV blinatumomab.
Gordon: Amgen: Current Employment, Current equity holder in publicly-traded company. Schwartz: Morphosys: Research Funding; Pfizer: Honoraria, Speakers Bureau; Gilead: Other: Travel grants, Speakers Bureau; Jazz Pharmaceuticals: Other: Travel grants, Speakers Bureau; Novartis: Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau; BTG International Inc: Membership on an entity's Board of Directors or advisory committees; MSD Sharp & Dohme: Membership on an entity's Board of Directors or advisory committees; Basilea: Other: Travel grants. Rossi: Celgene: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Honoraria; Abbvie: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; Alexion: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Huguet: Novartis: Other: Advisor; Jazz Pharmaceuticals: Other: Advisor; Celgene: Other: Advisor; BMS: Other: Advisor; Amgen: Other: Advisor; Pfizer: Other: Advisor. Hernández-Rivas: Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Wong: Amgen: Current equity holder in publicly-traded company; Amgen: Current Employment. Markovic: Amgen: Current Employment, Current equity holder in publicly-traded company. Katlinskaya: Amgen: Current Employment, Current equity holder in publicly-traded company. Panwar: Amgen: Current Employment, Current equity holder in publicly-traded company. Zugmaier: Micromet/Amgen: Patents & Royalties: Patents 20190300609 and 20130323247 licensed; receives royalties of family members of international applications published as WO2010/052014; WO2010/052013; WO2011/051307; WO2012/055961; WO 2012/062596; WO2014/122251; and WO2015/181683; Amgen: Current Employment; Amgen: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal